Abstract
Background: B-cell lineage cancers are a worldwide healthcare burden. Over 500,000 new cases of non-Hodgkin lymphoma (NHL) and 50,000 new cases of acute lymphoblastic leukemia (ALL) are diagnosed world-wide each year (seer.cancer.gov, Smith 2015, Solomon 2017). Despite progress in treatment, many patients diagnosed with this heterogeneous group of cancers still succumb to their disease. Recently approved autologous chimeric antigen receptor (CAR) T cells specific for CD19 have altered the treatment landscape for some patients with relapsed or refractory (R/R) B-cell malignancies, though significant toxicities associated with T-cell expansion and the necessity for bespoke manufacturing have limited their use. Natural killer (NK) cells, part of the innate immune system, efficiently recognize transformed cells and are particularly suited to address limitations of the approved CAR T products (Marcus 2014, Morvan 2016). Lacking a T-cell receptor and the consequent clonal expansion, non-engineered NK cells have been safely administered after lymphodepletion without side effects typically associated with T-cell therapies, such as severe cytokine release syndrome or neurotoxicity (Bachier 2020). Allogeneic NK cell-based therapies allow off-the-shelf use, obviating the necessity to wait for manufacture of autologous T-cell therapies. CD19-directed CAR NK cells have been administered safely, with promising preliminary efficacy (Liu 2020).
NKX019 is a cryopreserved product, composed of expanded NK cells engineered to express a humanized CAR against CD19, fused to co-stimulatory (OX40) and signaling (CD3ζ) domains to enhance their intrinsic antitumor activity. NKX019 also expresses a membrane-bound interleukin-15 (IL-15) to serve as an autocrine growth factor and thereby increase NKX019 persistence, with an in vivo half-life of over up to 28 days without systemic IL-2 support. Preclinical characterization has shown that NKX019 cells are 10 times more effective at killing CD19+ target cells than non-engineered NK cells, resulting in greater suppression of xenograft tumor models (Morisot 2020). Further, NKX019, unlike CD19 CAR T cells, retained cytotoxicity even when CD19 antigen density was reduced >50x on target cells. Hence, clinical evaluation of NKX019 is being undertaken in this Phase 1 study in subjects with R/R NHL or ALL.
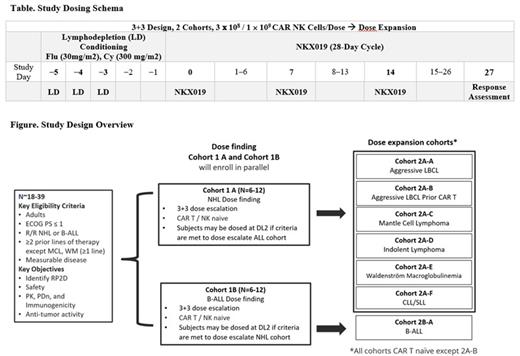
Methods: This is a multicenter, open-label, Phase 1 study of NKX019 (Figure). The study will be conducted in 2 parts: Part 1 (dose finding) to determine the recommended Phase 2 dose (RP2D) of NKX019 separately in adult patients with CAR T naïve R/R NHL or B-ALL, utilizing a "3+3" enrollment schema. Part 2 (dose expansion) will further evaluate safety and tolerability, pharmacokinetics (PK), immunogenicity, pharmacodynamics (PDn), and antitumor activity of NKX019 using RP2D with separate expansion cohorts for patients with ALL as well as different subtypes of NHL, including a cohort of CAR T pretreated large B-cell lymphoma. NKX019 is being manufactured from NK cells obtained from healthy adult donors. The study evaluates two dose levels of NKX019: 3 × 10 8 and 1 × 10 9 viable CAR+ NK cells. NKX019 will be administered on Days 0, 7, and 14 of a 28-day cycle following standard fludarabine/cyclophosphamide lymphodepletion (Table). Up to 5 total cycles may be administered based on response and tolerability assessed at the end of each cycle. The primary endpoint is incidence of adverse events, dose-limiting toxicities, clinically significant laboratory abnormalities, and determination of the RP2D. Secondary endpoints include evaluation of standard cellular PK parameters, PDn, immunogenicity, and antitumor responses. Subjects will be assessed for efficacy using disease-specific criteria: Lugano classification with LYRIC refinement for pseudo-progression (NHL), 2018 International Workshop (IW) criteria (CLL), 6th IW criteria (Waldenström macroglobulinemia [WM]), and National Comprehensive Cancer Version 1.2020 (B-ALL) (Cheson 2006, Cheson 2014, Hallek 2018, Owen 2013, Brown 2020). Enrollment across multiple sites in the US and Australia is expected to start in the second half of 2021.
Dickinson: Celgene: Research Funding; Gilead Sciences: Consultancy, Honoraria, Speakers Bureau; MSD: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria; Takeda: Research Funding; Amgen: Honoraria; Roche: Consultancy, Honoraria, Other: travel, accommodation, expenses, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau. Hamad: Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Bryant: Jansen, BMS/Celgene, Skyline Diagnostics: Consultancy; Amgen: Honoraria. Borthakur: Astex: Research Funding; University of Texas MD Anderson Cancer Center: Current Employment; Protagonist: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy; Ryvu: Research Funding; ArgenX: Membership on an entity's Board of Directors or advisory committees. Hosing: Nkarta Therapeutics: Membership on an entity's Board of Directors or advisory committees. Shook: Nkarta Therapeutics: Current Employment, Current equity holder in publicly-traded company. Tan: Nkarta Therapeutics: Current Employment, Current equity holder in publicly-traded company. Rajangam: Nkarta Therapeutics: Current Employment, Current equity holder in publicly-traded company. Liu: SITC: Honoraria; BMS; Karyopharm; Miltenyi: Research Funding; Agios; NGM Biopharmaceuticals; BeiGene: Consultancy. McSweeney: Kite-Gilead: Consultancy; Kite-Gilead, Autolus, Novartis: Research Funding; Kite-Gilead: Honoraria, Speakers Bureau. Hill: Novartis: Consultancy, Honoraria, Research Funding; Epizyme: Consultancy, Honoraria; AstraZenica: Consultancy, Honoraria; Beigene: Consultancy, Honoraria, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel Support, Research Funding; Pfizer: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria, Research Funding; Incyte/Morphysis: Consultancy, Honoraria, Research Funding; Gentenech: Consultancy, Honoraria, Research Funding; Celgene (BMS): Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding.